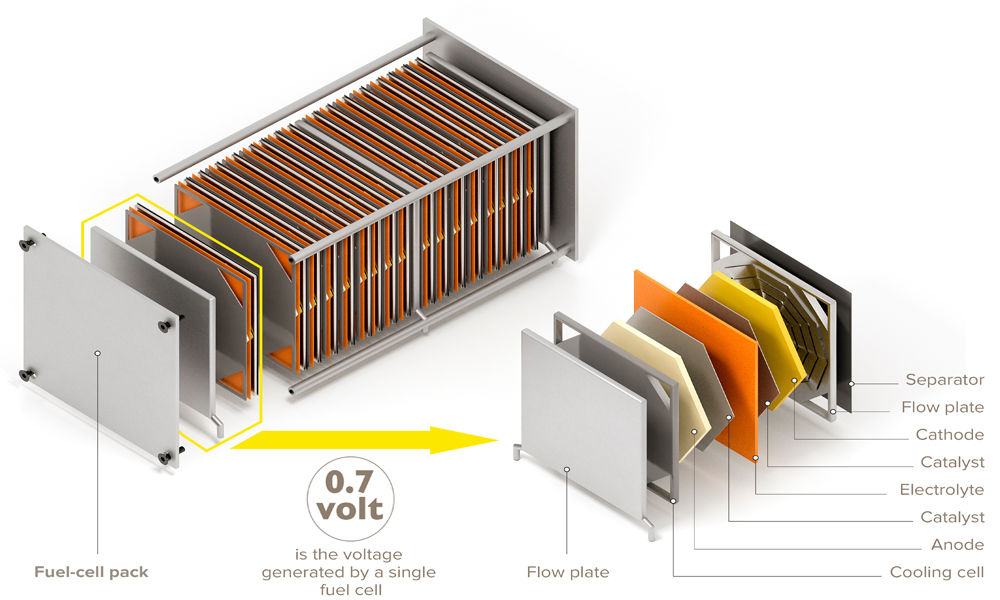
 A fuel cell uses a chemical reaction to produce electricity. It often uses hydrogen as the fuel, which combined with oxygen gives electricity, water, and heat as products. Fuel cells consist of a cathode, an anode, and an electrolyte. As the reaction proceeds, hydrogen ions move from the anode, through the electrolyte, to the cathode, creating an electric current. Catalysts are used to speed up the reaction. A fuel cell is an environmentally friendly technology that can continue to produce electricity so long as it is supplied with fuel and oxygen.
A fuel cell uses a chemical reaction to produce electricity. It often uses hydrogen as the fuel, which combined with oxygen gives electricity, water, and heat as products. Fuel cells consist of a cathode, an anode, and an electrolyte. As the reaction proceeds, hydrogen ions move from the anode, through the electrolyte, to the cathode, creating an electric current. Catalysts are used to speed up the reaction. A fuel cell is an environmentally friendly technology that can continue to produce electricity so long as it is supplied with fuel and oxygen.
MATERIALS
Porous carbon cloths and carbon fiber papers are typically used for the anode and cathode materials in fuel cells because they are lightweight, stable, and have high conductivity. These thin electrodes contain woven or arranged carbon fibers and allow current and gases to travel through the structure.
Often a catalyst is embedded in the electrodes. Most commonly, the catalyst is platinum nanoparticles. Since platinum is an expensive precious metal, systems that incorporate cobalt, iron, or nickel are being explored by many research groups.
The electrolyte component of the fuel cell allows transport of charged particles, usually hydrogen ions, across either a solid membrane or a liquid solution. If a liquid is used as the electrolyte, a solid separator is needed to allow transport of ions while preventing contact between the anode and cathode. While liquid electrolytes transport ions more effectively in some systems, they have the potential to leak, which can be a safety hazard.
Fuel cells are categorized by the type of electrolyte they employ.
- Alkaline Fuel Cells use potassium hydroxide solutions as electrolytes. These require pure hydrogen and oxygen as reactants. Inexpensive polymer films, such as polypropylene, can be used. Alkaline fuel cells are extremely efficient and produce water pure enough for drinking.
- Proton Exchange Membrane Fuel Cells, also called polymer electrolyte membranes, are lightweight and contain no liquids, using Nafion as a solid electrolyte to simultaneously transport ions and separate anode from cathode. These fuel cells operate at low temperatures and are very safe, although their cost of materials can be high.
- Phosphoric Acid Fuel Cells contain high concentrations of liquid phosphoric acid within a silicon carbide matrix for their electrolyte. While able to accept a wider variety of fuels, these cells have the limitations of low power output and high cost.
- Molten Carbonate Fuel Cells use a salt carbonate solution as the electrolyte with lithium aluminium oxide as a ceramic separator. These systems operate at high temperatures and can use non-platinum catalysts such as nickel.
- Solid Oxide Fuel Cells are unique in using a ceramic metal oxide as the electrolyte. Because of very high temperature operation, materials must be studied and chosen carefully for these fuel cells in order to avoid cracks and damage.
- Direct Methanol Fuel Cells can use methanol as the fuel instead of hydrogen gas, making fuel storage much easier. This reaction creates carbon dioxide as an additional byproduct. These are generally low temperature, low power systems that often use polymer electrolyte membranes.
APPLICATIONS
Fuel cells are used in transport applications such as trains, cars, and buses; electronics such as laptops, cell phones, and cameras; and stationary power supplies such as telecommunications and water heating.
References
- “Fuel Cells.” National Museum of American History, Smithsonian Institution. 1990.
- https://americanhistory.si.edu/fuelcells.htm
- Zhang, X, Shen, Z. “Carbon Fiber Paper for Fuel Cell Electrode.” Fuel. 2002, 81, 2199-2201.
- “Batteries and Fuel Cells.” Energy Materials Center, Cornell University. 2013.
- https://www.emc2.cornell.edu/content/view/membranes.html
