Electrocatalysis
Chemical reactions are often limited by the amount of time it takes, and the amount of energy required for the reactants to form the products. A catalyst is a compound that can increase the speed at which reactions occur, and lower the energy required for the reaction to take place. One of the key features of a catalyst is that it is not consumed by the chemical reaction and can be re-used in subsequent reactions. Electrocatalysis is an electrochemical reaction that takes advantage of a catalyst. 1
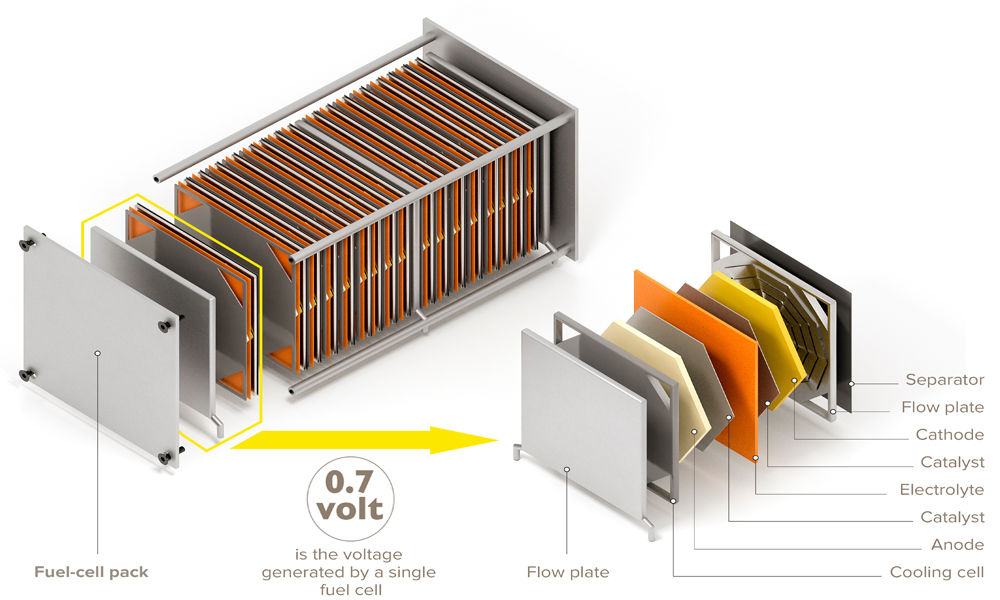
The most prevalent example of this is in fuel cell design. Fuel cells are an electricity storage and use platform where electricity can be stored in chemical bonds. A great example of this is with hydrogen gas, comprised of two hydrogen atoms that can be broken down to two protons and two electrons. A catalyst at an electrode surface can take an electrical current, along with protons in solution, and form hydrogen gas. A good catalyst will do this more quickly, and require less energy. 1 On the other hand, if we wanted to use the electrons stored in the hydrogen bond, we could use a catalyst at an electrode surface, and retrieve our stored electricity.
This research in energy storage is especially important in our attempt to use more green energy sources such as solar panels and wind turbines. While the sun doesn’t always shine, and the wind doesn’t always blow, the current power grid design calls for the availability of electricity at all times. It is important to not only optimize these catalytic methods for energy storage, but find ways to properly implement them for green energy storage.
Platinum catalysts are often used for energy storage and use, but it has been shown that very little of these platinum catalysts are being used. 2 This is because platinum catalysis occurs at the surface of platinum when the reactant molecule adsorbs to the surface. One method for increasing the catalytic production would be to increase the amount of available surface area per catalyst present. These catalysts can be further optimized by introducing a support material such as carbon nanotubes, or various nanowires, where platinum nanoparticles are disbursed along the carbon nanotube. 2 This is largely due to the fact that with the platinum nanoparticles, there is a larger ratio of surface area to total volume of platinum, and with the support material the catalyst is more spread out, thus increasing the catalytic properties.
Photocatalysis
Like electrocatalysis, photocatalysts can lower the energy required for a reaction to occur, and increase the speed at which a reaction occurs. Unlike the electricity used in electrocatalysis, photocatalysis harnesses the energy of light. When light with a wavelength that has high enough energy interacts with a material, an electron can be excited to the conduction band, where it can more easily be removed.3
Photocatalysis shows promise for use in photoelectrochemical cells, where solar energy conversion can be directly stored. TiO2 was the first material used for photoelectrochemical water splitting, where H2O is split to form H2 and O2 gases for energy storage. 3 Just like the electrocatalysts, the performance of these photocatalysts depends on surface reactivity. In order to increase performance of these photocatalysts, the size has been brought down to a nanoscale range, in order to increase the ratio of surface coverage to volume of catalyst. 3 The major problem with TiO2 is that the light that it absorbs is UV, which is less than 5% of the total light energy that the sun emits. 3 One method for modifying these particles is doping, where an impurity that is introduced can shift the wavelength of light that the particle absorbs. 3
Another method to improve the efficiency and efficacy of these photocatalysts is by forming composites of multiple photocatalysts. 3 This can combine properties of each photocatalyst, and often also creates new properties. 3 The addition of a plasmonic metal into the catalyst design can also increase performance.
Photocatalyst research is heavily focused on increasing stability and implementation in water splitting. Optimization of efficiency and decrease in required energy are also key components of photocatalysis research. 3 Like electrocatalysts, it has been demonstrated that distributing photocatalysts on a support such as carbon nanotubes or graphene can increase performance optimization. 3
References
(1) Ramaswamy, N.; Mukerjee, S. Advances in Physical Chemistry 2012, 2012, 1–17.
(2) Vignarooban, K.; Lin, J.; Arvay, A.; Kolli, S.; Kruusenberg, I.; Tammeveski, K.; Munukutla, L.; Kannan, A. M. Chinese Journal of Catalysis 2015, 36, 458–472.
(3) Li, J.; Wu, N. Catalysis Science & Technology 2015, 5, 1360–1384.
