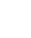How do we know what the temperature of our planet was a million years ago, to better understand climate change? Where did Őtzi the Iceman, Europe’s oldest mummy, live as a child and an adult? What evidence gives doping agencies the gold standard to determine whether testosterone in an athlete’s sample is synthetic? How do we obtain 3D images of tumors in soft tissues? The answers to all of these questions can be revealed through a deeper understanding of isotopes of the elements. IUPAC Periodic Table of the Elements and Isotopes (IPTEI), the interactive IPTEI and supporting “Isotopes Matter” educational resources (created through IUPAC Projects 2014-024-1-200 and 2007-038-3-200) aim to provide answers to these questions and many more by educating individuals on the importance of isotope science.
1Introduction
2What do you think of when you hear the word isotope?
Perhaps words such as “dangerous”, “radioactive”, “pollution” or “atomic bomb” come to mind? Or do you think of “essential”, “lifesaving”, or “beneficial”? A decade ago, a joint IUPAC project of the Inorganic Chemistry Division (Division II) and the Committee on Chemistry Education (CCE) was established to provide educational outreach to challenge the thinking of students, teachers, and the public about the importance of both stable and radioactive isotopes in everyday life. The result of this project was the IUPAC Periodic Table of the Elements and Isotopes (IPTEI), a new periodic table that not only shows the traditional information about the 118 elements, but also their many isotopes and applications. The uses of these isotopes in the following scientific areas are highlighted:
- Biology
- Earth and planetary science
- Forensic science and anthropology
- Geochronology (including isotopic dating of materials)
- Industry
- Medicine
- Sources for radioactive isotopes
As more research in the field of isotope science is done, the areas of application for knowledge about isotopes are sure to broaden and expand. However, many common misconceptions about isotopes still exist in the scientific and educational communities, as well as the general public. The intention of the IPTEI and accompanying resources is to address these misconceptions and to educate about the many benefits and applications of the use of isotopes.
3How is the IPTEI different from IUPAC periodic tables of the past?
When looking at the IUPAC Periodic Table of the Elements and Isotopes for the first time, you may notice that the colour-coding of the element tiles doesn’t match colour schemes from most periodic tables found in chemistry textbooks or hanging on your classroom wall. Instead, the pink, blue, yellow, and white backgrounds for each element tile represent the element’s number of stable isotopes and the variability of their relative abundance. The atomic weights of each element are represented differently depending on the colour of the element as well.
White backgrounds, such as radium, illustrate elements that have no standard atomic weight because all of its isotopes are radioactive and no isotope occurs with a characteristic isotopic abundance.
Blue backgrounds, such as gold, are used to demonstrate elements with a single isotope used to determine its standard atomic weight, which is displayed as a single number with an IUPAC evaluated uncertainty.
Yellow backgrounds, such as barium, represent elements with two or more isotopes used to determine its standard atomic weight. Their standard atomic mass is also given as a single number with an IUPAC evaluated uncertainty because the range of their variation has either not been determined or is too small to require upper and lower bounds.
Pink backgrounds, such as sulfur, are used for elements having two or more isotopes that are used to determine its standard atomic weight; the isotopic abundances and atomic weights vary in normal materials, and these variations are well known, and the standard atomic weight is given as lower and upper bounds within square brackets, [ ]. Conventional atomic weights, for necessary calculations in science and industry when the origin of the sample is unknown, are shown in white.
Each element’s slot on the periodic table also features a pie chart which acts as a visual representation of the element’s isotopic composition. The pie chart visually reinforces that not all atoms of an element are the same.
4What information will I find on the interactive IPTEI?
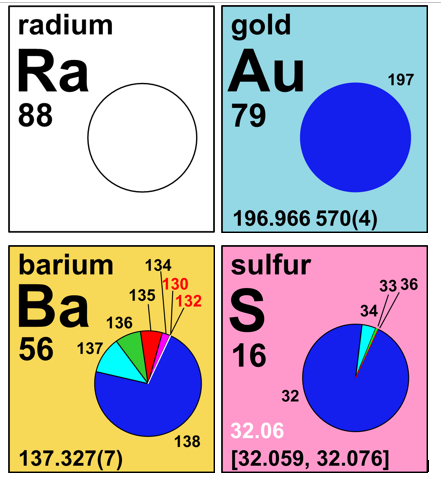
Besides containing all information included on the print version of the IPTEI, the online interactive IPTEI allows users to interact with additional information about each element and its isotopes. Users can click on any element to view an interactive information card containing enlarged pie chart. Clicking on each isotope reveals its percent abundance and atomic mass. The cards also contain links to a more detailed element by element summary of additional applications and a chart of all stable and radioactive isotopes for that element. On average, there about ten times as many radioactive isotopes as stable isotopes and most of these are not included in the calculated atomic weight because of their radioactive lifetime.
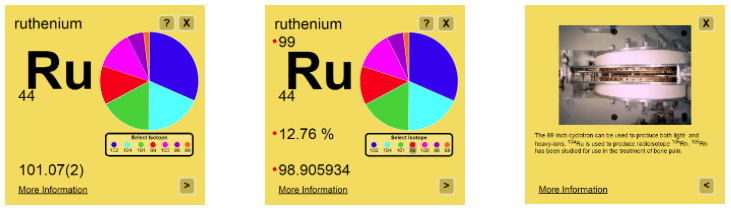
On the back side of the interactive cards is an interesting real-world example or fact related to applications of isotopes of the selected element. These examples illustrate the wide range of applications of isotope science and encourage the user to consider other possible applications of isotopes.
5How were the print and interactive IPTEI created?
Building on work done by the Commission on Isotopic Abundances and Atomic Weights (CIAAW) , the project began back in 2007 with a project proposal to create a periodic table for the educational community. This periodic table would emphasize the existence of isotopes and how their number of isotopes and their relative abundances determine atomic weights. Another goal was to highlight the diverse applications of isotopes in chemistry and many other sciences. The print version of the original IUPAC Periodic Table of the Elements and Isotopes, along with supporting material for each isotope was created.
Six years later, a second joint project created an interactive electronic version of the IPTEI and a set of supporting educational materials called “Isotopes Matter”. The interactive version of the periodic table and the Isotopes matter resources were developed by the King’s Centre for Visualization in Science (KCVS) in collaboration with IUPAC scientists and educators.
The King’s Centre for Visualization in Science (KCVS) is an interdisciplinary research centre of the King’s University in Edmonton, Canada. Interdisciplinary teams of undergraduate students have played a key role since the establishment of KCVS in 2005, working with the centre directors to create interactive learning resources that each year enable hundreds of thousands of students, educators, and the public from over 100 countries see and understand science better.

If you would like to see a personal account of the development of the IPTEI, click here.
6Are atomic weights constants of nature?
Consider this: water is made up of three atoms, one oxygen atom and two hydrogen atoms. Most of the time, these atoms are the oxygen-16 isotope and two hydrogen-1. However, a few of the water molecules will contain an oxygen-18 or deuterium (hydrogen-2) atom, resulting in a slightly heavier water molecule. These heavier water molecules will less readily evaporate, and when they do, they’re the first to condense. Because of this, the ratio of heavier to light water molecules in precipitation will decrease as the temperature decreases.
If you were to extract and measure the atomic weight of oxygen atoms in water molecules obtained from precipitation formed at low temperatures, you would get a smaller value than if the water sample had precipitated at higher temperatures. Getting two different values for an atomic weight can only mean one thing: it’s not a constant of nature.
You may have noticed that the pink elements on the IPTEI do not have a single number for their atomic weight, but rather an atomic weight interval in square brackets. This is because for any element that has two or more stable isotopes, there is always the possibility that the relative amounts of the stable isotopes may differ from one sample to another found in nature.
Because mass spectrometry uses electric and magnetic fields to measure the charge to mass ratio of molecules in a sample of ionized molecules, the heavier isotopes will separate from the lighter isotopes, and detectors can determine the mass of each isotope and its relative abundance.
The interactive Isotopes Matter suite of resources includes demonstrations of methods such as mass spectrometry and features lessons and applets that provide demonstrations of applications and evidence regarding the nature of atomic weights.
References
- King’s Centre for Visualization in Science, “Isotopes Matter” educational resources including the Interactive Electronic Periodic Table of the Elements and Isotopes - https://www.isotopesmatter.com